Abstract
Introduction: Hematologists frequently face a percentage of patients with a mild bleeding tendency due to a haemostatic abnormality that cannot be identified with conventional laboratory techniques. Such patients are termed as having an unclassified bleeding disorder (UBD). A good diagnosis is important in order to prevent bleedings during invasive processes and/or childbirth by choosing the optimal therapeutic treatment.
We aimed to investigate hemostatic parameters that may be altered in patients with UBD in order to determine the cause of their bleeding symptoms. In particular, possible defects in the tissue factor (TF)-mediated regulation of coagulation or in the plasmin generation during the fibrinolysis, as well as the possible beneficial effects of treatment with antibodies blockers of TFPI.
Methods: This is a single-centre, case-control, non-interventionist, prospective study.
During an 8 months-period, 40 patients with bleeding symptoms (evaluated with ISTH-BAT score) were studied. Routine coagulation tests (aPTT and PT) and platelet function testing [aggregometry, PFA-100, flow cytometry and Total Thrombus-formation Analysis System (T-TAS; Zarcos, Japan)] were performed. In 17 patients, no abnormalities were detected in platelet function and/or in coagulation tests; so the following procedures were performed:
Thrombin generation test by Calibrated automated thrombography (CAT) in samples of platelet poor plasma with corn trypsin inhibitor (CTI), an inhibitor of contact activation phase, using a low amount of TF (1 pM TF and 4 µM phospholipids) as a trigger to allow the evaluation of the TF-dependent pathway.
Plasmin generation (PG) test with a kit from Synapse Research Institute (Maastricht, The Netherlands), using Thrombinoscope software.
TFPI activity in plasma, measured with ACTICHROME® TFPI kit (Biomedica Diagnostics, USA).
The effects of rFVIIa (Novoseven, NovoNordisk; 90 µg/kg) and of a human Anti-TFPI recombinant Ab (clon mAb2021, Creative Biolabs; 400 ng/ml) were tested in CAT, PG and TFPI activity tests.
Results: Those patients with aPTT, PT and a platelet function within normal range were further studied performing thrombin generation, plasmin generation and TFPI activity tests.
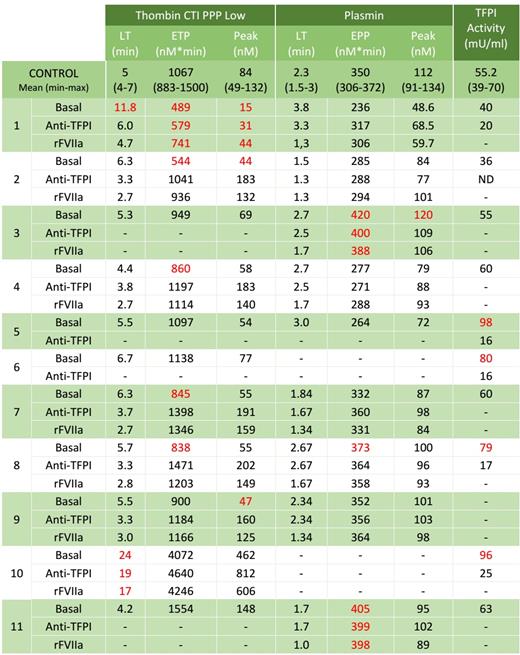
Table 1 shows the results obtained.
Samples from patients 1, 2, 4, 7, 8, 9 and 10 had a diminished generation of thrombin, and in vitro treatment with anti-TFPI and rFVIIa only ameliorated thrombin generation in samples from patients 4, 7, 8 and 9. Plasma from patients 8 and 10 had increased activity of TFPI.
Generation of thrombin in samples from patients 3, 5, 6 and 11 was within normal range. Plasmin generation was increased and not modified by in vitro treatment with anti-TFPI and rFVIIa in samples 3 and 11; whereas samples 5 (with normal plasmin generation) and 6 (with no data of plasmin generation due to lack of enough sample) had a high TFPI activity in plasma that was inhibited by anti-TFPI.
Normal values in all these parameters evaluated were found in six patients, indicating the involvement of different mechanisms that are still unknown.
Conclusions: UBD have a diverse pathological basis for the bleeding. So, a single laboratory test to make a correct diagnosis of this pathology cannot be recommended. In accordance with this fact, a personalized treatment should be applied for each patient.
Non-conventional laboratory tests need to be standardized and included for studying possible defects in the regulation of TF and/or plasmin pathways that can be involved in very rare mild bleeding phenotypes.
TFPI inhibition might emerge as a good therapy for some of these patients.
Failure to detect the bleeding cause in some of these patients, suggests the need to perform further studies in this field.
This work was supported by Novo Nordisk Pharma S.A.
Table 1- Thrombin and plasmin generation and TFPI activity in samples of patients with UBD. Results out of normal range are shown in red.
LT: lagtime; ETP: endogenous thrombin potential; EPP: endogenous plasmin potential; TFPI: Tissue factor pathway inhibitor.
Alvarez Román: Grifols: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; CSL-Behring: Consultancy, Honoraria, Research Funding; Biomarin: Consultancy, Honoraria, Research Funding; Novo-Nordisk: Consultancy, Honoraria, Research Funding; Octapharma: Consultancy, Honoraria, Research Funding; Sobi: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding. Martín: Novo Nordisk: Speakers Bureau; Pfizer: Speakers Bureau. Jiménez-Yuste: F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; BioMarin: Consultancy; Takeda: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Research Funding; Sobi: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; CSL Behring: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Octapharma: Consultancy, Honoraria, Research Funding; NovoNordisk: Consultancy, Honoraria, Research Funding; Grifols: Consultancy, Honoraria, Research Funding. Canales: Eusa Pharma: Consultancy, Honoraria; Sandoz: Honoraria, Speakers Bureau; Sanofi: Consultancy; Karyopharm: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Incyte: Consultancy; Gilead/Kite: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Speakers Bureau; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; iQone: Honoraria; Celgene/Bristol-Myers Squibb: Consultancy, Honoraria. Butta: Novo-Nordisk: Speakers Bureau; Takeda: Research Funding, Speakers Bureau; Roche: Speakers Bureau; CSL-Behring: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal